Nova Biomedical Launches New Laboratory Blood Glucose Reference AnalyzerBy HospiMedica International staff writers[19 Nov 2020] 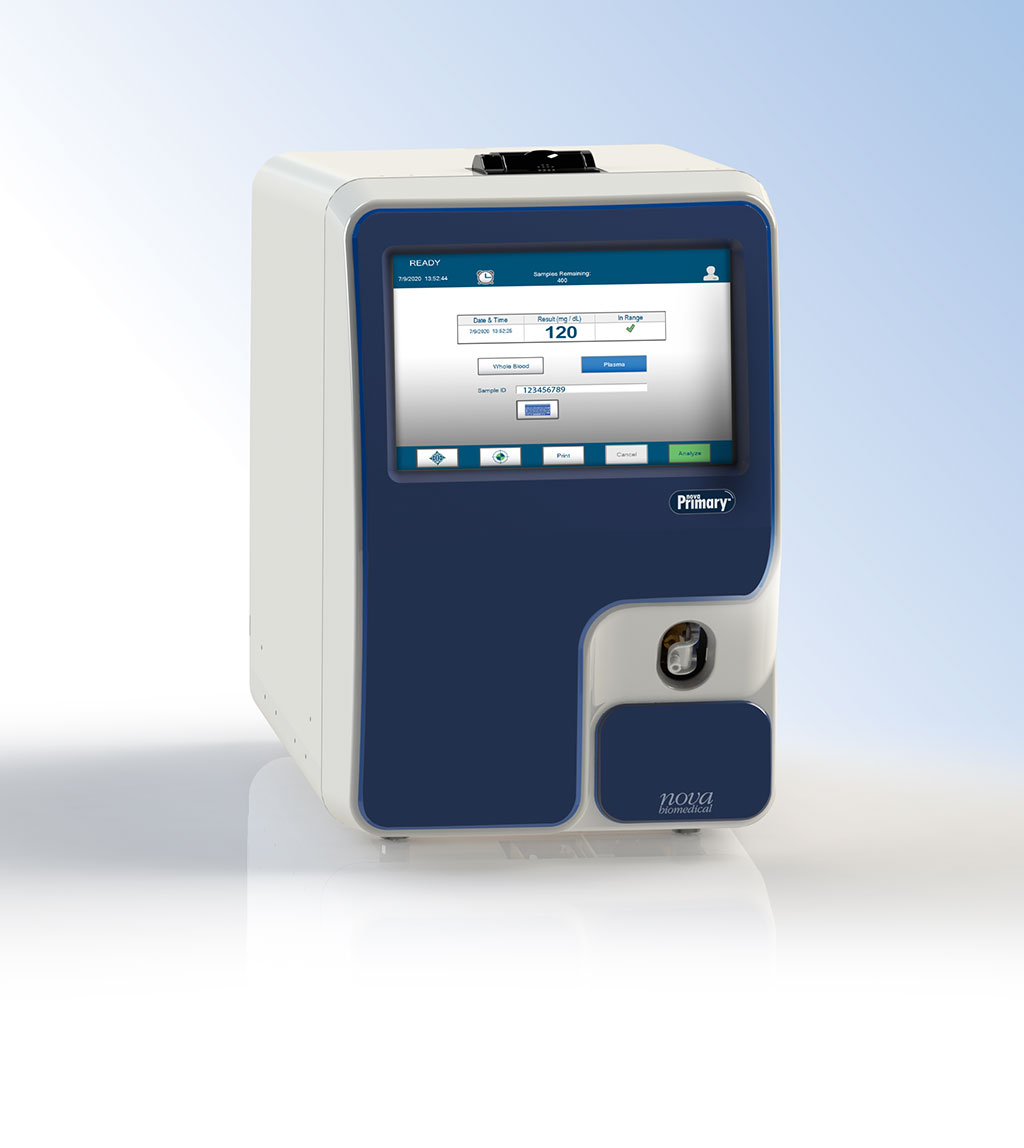 |
|
| Nova Biomedical (Waltham, MA, USA) has launched Nova Primary, a rapid, accurate, easy to use, blood glucose laboratory analyzer that fills the need for a new glucose reference analyzer to replace the YSI STAT PLUS 2300 Glucose and L-Lactate analyzer from YSI, Inc., (Yellow Springs, OH, USA). Manufacturers of blood glucose measuring devices and clinical diabetes researchers have relied on the YSI 2300 as a reference and correlation analyzer. However, as of July 2021, YSI, Inc. is no longer supporting the analyzer, and its discontinuation has left a critical industry void. The Nova Primary analyzer fills that need and will be available for sale in CE regulated countries from January 2021. The Nova Primary was also submitted to the US Food and Drug Administration (FDA) for 510(k) clearance in November 2020 and is anticipated to receive clearance in the first half of 2021. Similar to the YSI 2300, Nova Primary uses a single, reusable glucose electrochemical sensor based on glucose oxidase and has a measurement range of 10-900 mg/dL. It uses a small, 25 microliter venous whole blood or plasma sample which is internally diluted like the YSI. Results are available in approximately two minutes. Nova Primary’s large color touchscreen display and intuitive, icon-based graphical user interface make it very straightforward to use. A single calibrator pack uses RFID data management to monitor the pack expiration date and number of samples remaining, eliminating the need for separate reagent bottles and daily monitoring of their levels. In clinical laboratory studies using venous whole blood and plasma Nova Primary demonstrated excellent correlation to the YSI 2300 and is traceable to NIST glucose standards. |
|